Motivation
Important but unknown things are worth further studying.
An intrinsically disordered region (IDR) of a protein is a segment that lacks a stable or recognizable 3D structure. These parts of proteins are hard to predict with even the best neural networks, because they have high entropy (large amount of possible shapes they can be in, depending on noise and presence of binding partners) — hence “disordered”. This is a big challenge to the biological paradigm, because in the past century people rely on distinct structures to match to the function of the protein (to bind to other proteins, to fold or contract or inhibit/disinhibit or signal.
IDRs important but unknown. Plus, they are super common.
Since 2015, people in the field of soft and living matter have proposed theories about them. However, there is not as much experimental evidence supporting one theory‘s prediction over another.
It would be exciting to see what evidence comes out of our experiment with the IDR of the protein Canoe.
The above is what I think I can say to the research community. Laying these out as axioms help me address my skepticism and join the conversation. Personally, I find the transient and nuanced shape of the IDR worthy enough of attention and interaction.
Question
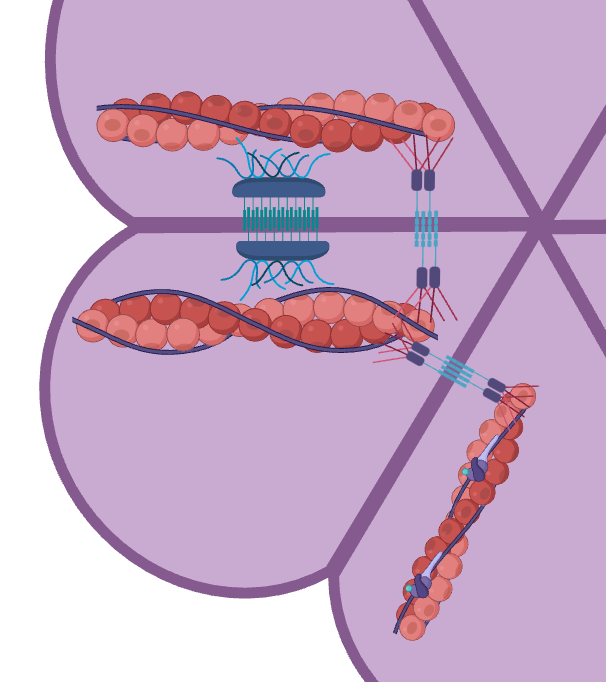
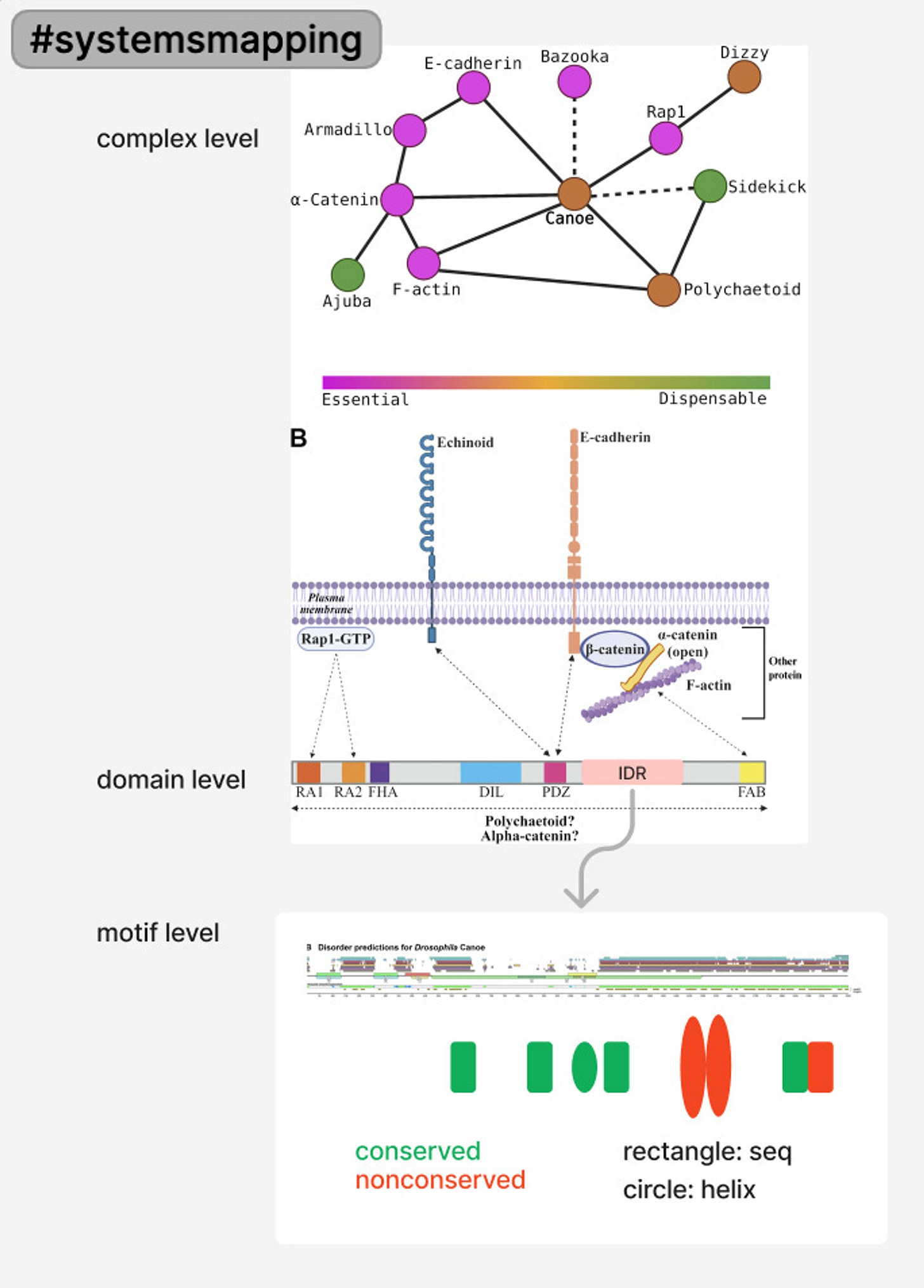
Canoe is a large scaffold protein in a multivalent protein network that comprises the link between the cytoskeleton in cells and the adhesion junction between cells.
scaffold: a functional category of proteins that are not necessary but sometimes enhance the binding of necessary proteins (junction and cytoskeleton are both necessary proteins in the task of linking cells & staying alive).
multivalence: a state of a protein having many potential binding partners and bonds.
What is the function of the IDR in the protein Canoe?
Personally, I want to know how gaps at the cell-cell level emerge into defects at the tissue level. Is it by feedback or something else? What is a useful metaphor? How can we get evidence that tell the metaphors apart?
Hypothesis
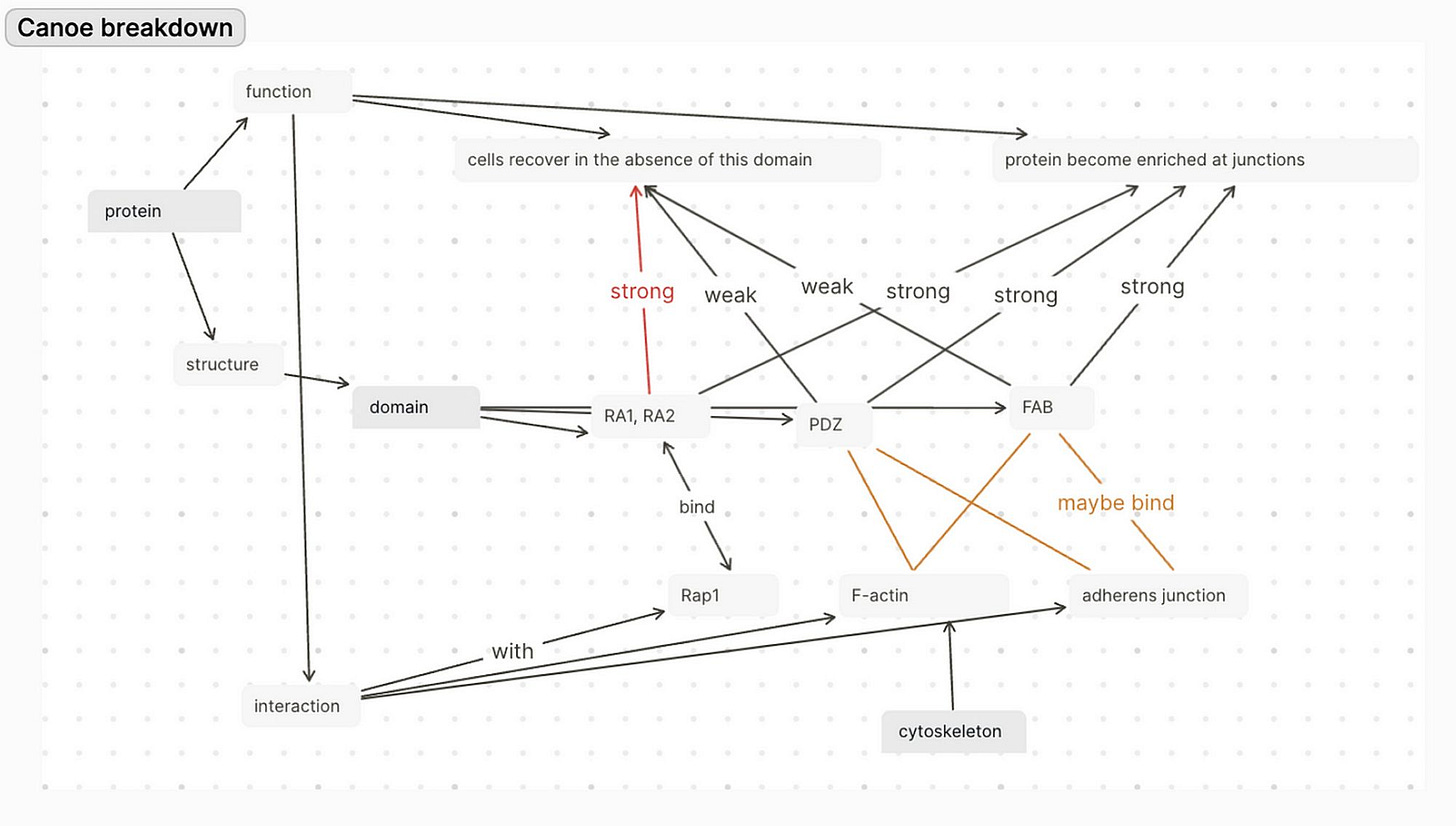
The potential ways that the Canoe IDR can have sufficient/standing effect on the organism are mainly at the protein interaction level. As in, the IDR might affect the binding between Canoe and other proteins in the linkage network joining the cytoskeleton that make forces in the cell and the adhesion junctions linking cells together as they change shape in a group. IDR could be contributing to protein-protein interaction in the following ways:
length
binding
sensing curvature (Zeno, 2018)
converting the mechanical signal into a biochemical signal
Methods
model system: Drosophila embryo
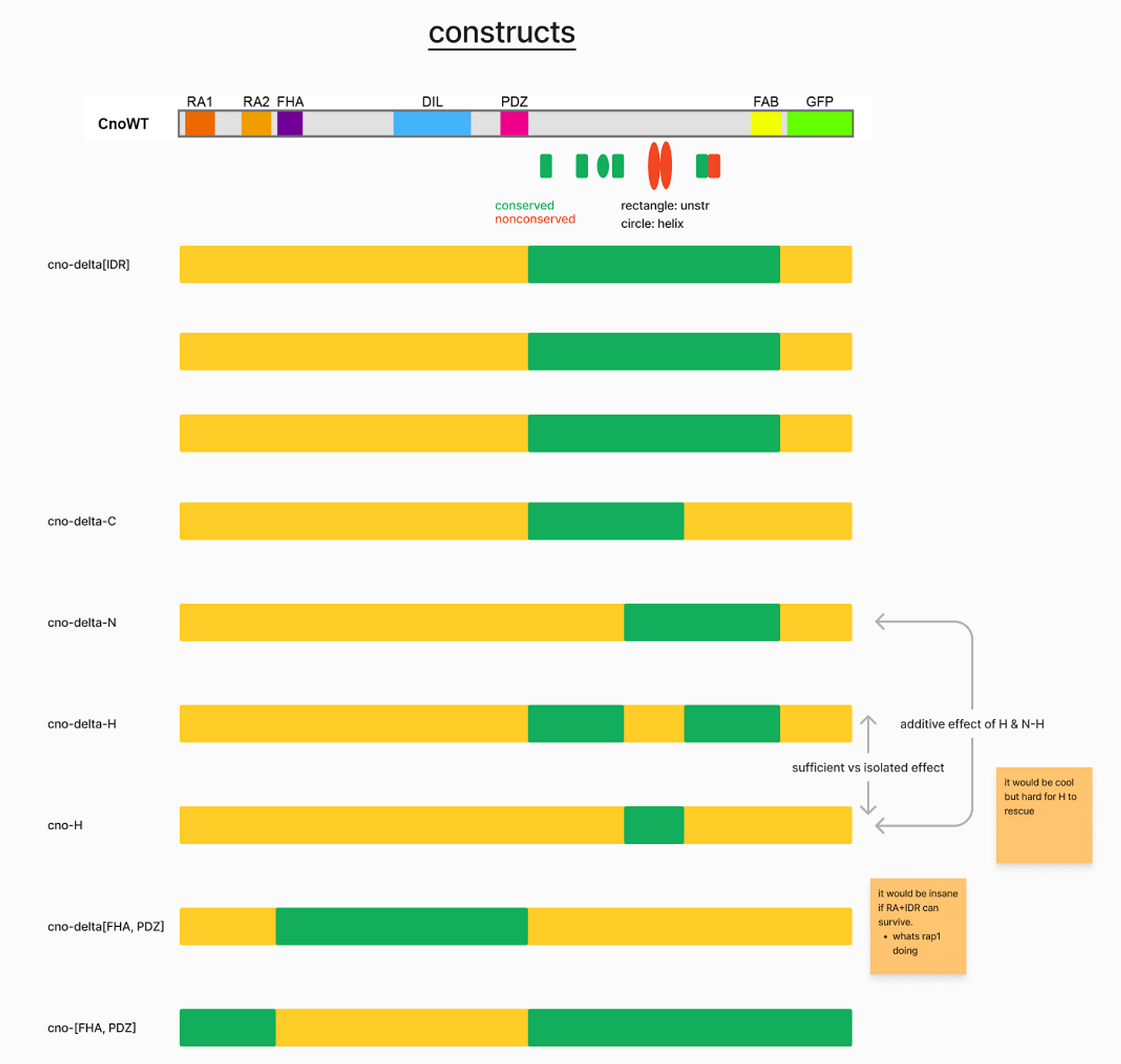
statistical inference: predicting which DNA makes our protein region, which parts of the region could be most most responsible for the emergent properties
genetics: delete and/or modify genes to delete and/or modify proteins. (Note: the assumption in this method is that isolated DNA mutation → isolated Protein structure change → protein function change in force generation → phenotype change → essentiality of the gene).
biochemistry: what is the real structure of the protein? which proteins interact?
cuticle phenotyping: how severe is the tissue failure?
confocal microscopy: how well do the proteins aggregate at the locations where cells experience most tearing force as the embryo folds?
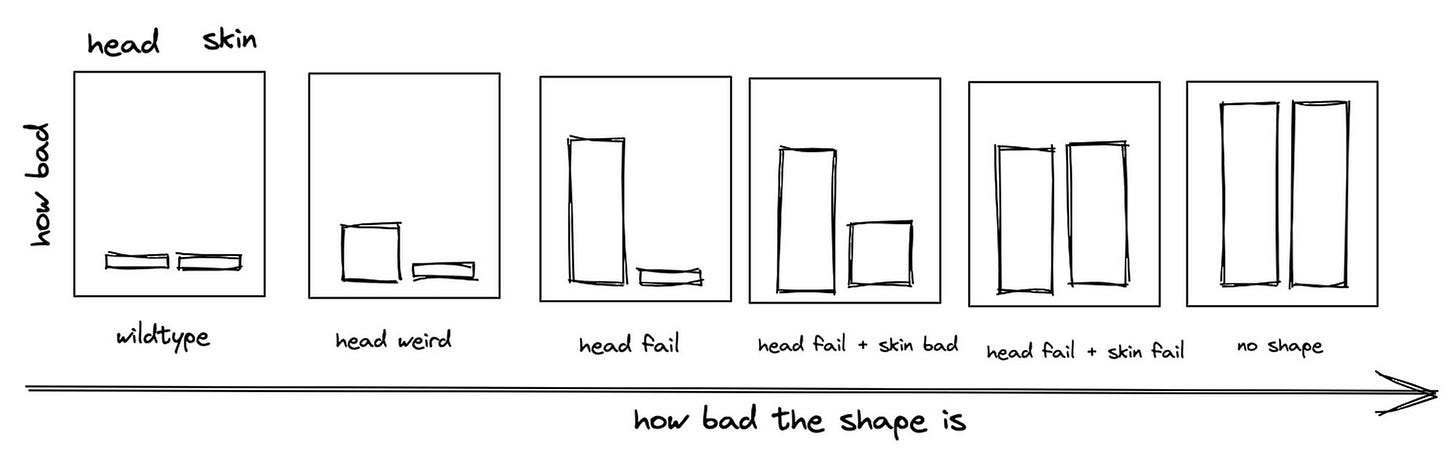
Expected Results
References
Gurley, N., Szymanski, R., Dowen III, R. H., Butcher, T. A., Ishiyama, N., & Peifer, M. (2023). Exploring the evolution and function of Canoes intrinsically disordered region in linking cell-cell junctions to the cytoskeleton during embryonic morphogenesis. PLosONE, 2023-03.
Perez-Vale, K. Z., Yow, K. D., Johnson, R. I., Byrnes, A. E., Finegan, T. M., Slep, K. C., & Peifer, M. (2021). Multivalent interactions make adherens junction–cytoskeletal linkage robust during morphogenesis. Journal of Cell Biology, 220(12), e202104087.
Proulx, S. R., Promislow, D. E., & Phillips, P. C. (2005). Network thinking in ecology and evolution. Trends in ecology & evolution, 20(6), 345-353.
Zeno, W. F., Baul, U., Snead, W. T., DeGroot, A. C., Wang, L., Lafer, E. M., ... & Stachowiak, J. C. (2018). Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing. Nature communications, 9(1), 4152.
Philosophy
(This section is summarized from my draft by GPT. I am too sleepy to catch errors for now. Will check!)
Defining 'Disordered' Protein Structure
In the realm of protein science, characterizing and defining the nature of protein structures is paramount. One critical distinction is between structured and disordered protein regions.
Structured Proteins are those with a well-defined three-dimensional (tertiary) structure. These structures are stable over time and are resistant to external forces and changing concentrations. Within structured proteins, we can identify secondary structures, such as alpha helices and beta sheets, which are specific arrangements of amino acids that provide stability and functionality.
Disordered Proteins or Intrinsically Disordered Regions (IDRs), on the other hand, lack this stable tertiary and secondary structure. Instead, IDRs exist as an ensemble of conformations, which can vary over time or in response to specific conditions. This intrinsic disorder does not mean chaos but rather flexibility. It allows proteins to adopt various shapes and interact with multiple partners, adapting to different cellular tasks.
The distinction between structured and disordered regions is significant because it affects a protein's functionality. Structured regions usually have a well-defined role, while IDRs often engage in dynamic, context-dependent interactions. Their disorderliness doesn't imply inefficiency; in fact, it can be a strategic advantage, allowing proteins to perform multiple functions and participate in various cellular processes.
The analogy: Understanding the concept of 'disordered' structure in proteins often relies on analogies and comparisons to more familiar terms like 'structured' or 'ordered.' Just as we grasp complex ideas by drawing parallels to simpler ones, the protein structure can be simplified through analogy. It's akin to recognizing a piece of music: structured proteins have a clear melody, while disordered regions play a more improvisational tune. In scientific terms, structure refers to the spatial arrangement of a protein's atoms, like the notes in a musical composition. Structured proteins possess a stable, three-dimensional form that endures over time, akin to a well-composed symphony. On the other hand, disordered regions lack this definitive structure; they're more like a jazz improvisation, flexible and adaptable to different situations. While they may lack a stable form, these disordered regions are vital to the orchestra of cellular processes, participating in a dance of molecular interactions. Therefore, the concept of protein structure, whether ordered or disordered, becomes clearer through analogy, making it accessible to scientists and non-scientists alike.
Using Network Models to Understand Disordered Protein Structures
In recent years, scientists have turned to network models to gain deeper insights into the roles of intrinsically disordered regions (IDRs) within proteins.
Protein Interaction Networks offer a powerful framework for understanding the complexity of biological systems. In these networks, proteins are nodes, and interactions between them are edges. This metaphor provides a way to conceptualize how proteins communicate and collaborate within the cell.
The Redundancy Principle: One key insight from network models is the redundancy principle. This principle suggests that the presence of multiple proteins with similar functions in a network can confer robustness to the system. This redundancy means that if one protein fails or is altered, another can often step in to perform the same function. IDRs play a significant role in this redundancy, as their structural flexibility allows them to interact with multiple partners, contributing to the resilience of biological systems.
Flexibility and Adaptability: IDRs' intrinsic disorder allows them to adapt to different cellular tasks by interacting with various partners. This adaptability is crucial in dynamic cellular processes where proteins need to switch roles quickly.
Beyond Traditional Structure-Function: Network models also challenge the traditional one-to-one correspondence between protein structure and function. They highlight that IDRs, despite their lack of stable structure, can have multifaceted functions within the cellular context.
The analogy: Analogical thinking plays a crucial role in the realm of biology, where complex systems like proteins are often understood through comparisons to more familiar concepts. One powerful analogy involves viewing proteins as networks. In this analogy, proteins become nodes, and their interactions resemble the connections between nodes in a network. This comparison offers sensory and logical connections. Sensory, because physical relations between proteins mirror the tangible links in a network, and logical, because these relations can be discrete and local, much like the connections between nodes. The network analogy allows us to simplify the intricate web of protein interactions into a comprehensible model. By breaking down the complexity of protein behavior into a network structure, we can analyze how disordered protein regions fit into this larger picture. This analogy, rooted in both sensory and logical resemblances, serves as a valuable tool for scientists seeking to unravel the roles and functions of disordered protein structures within the cellular environment.
I am trying to imagine myself as the intrinsically disordered region of a protein. A string of amino acids dancing around. I am already quite chaotic and heated so it’s easy to relate. I wonder when they cool down and commit to their binding partner. Is it a marriage? They usually perform many roles in the cell though. It’s an interesting philosophy that they live by.
I got lured by the English word “network” and “molecular machine” to see the protein as a technology.
“I want to write a story of a day in life in the future when you can use a pen to draw something then it becomes alive, as protein.”
“you mean, a farmer?”
“oh I guess…”
This is something I wanted to append to the essay yesterday. Firstly I don’t have to work to get the belonging I need. Secondly belonging takes knowledge and time — for example if you want to live on a land project you need to know the climate, the vegetables, the cycles, the accidents, the people. Thirdly I could work for people I care about while still caring for myself through pleasure and learning. For example I could research — gather information and synthesize knowledge — for the farmers I have befriended all these years, including my grandma. No matter what format, I would like to experience life together through interacting with and making stuff.